Table of Contents
● One-Year Clinical Results of LuX-Valve Plus TRAVEL II Study were Officially Released at TCT 2024 in U.S.
● One-Year Outcomes of LuX-Valve Plus in Large Anatomy Patients were Shared at PCR London Valves 2024
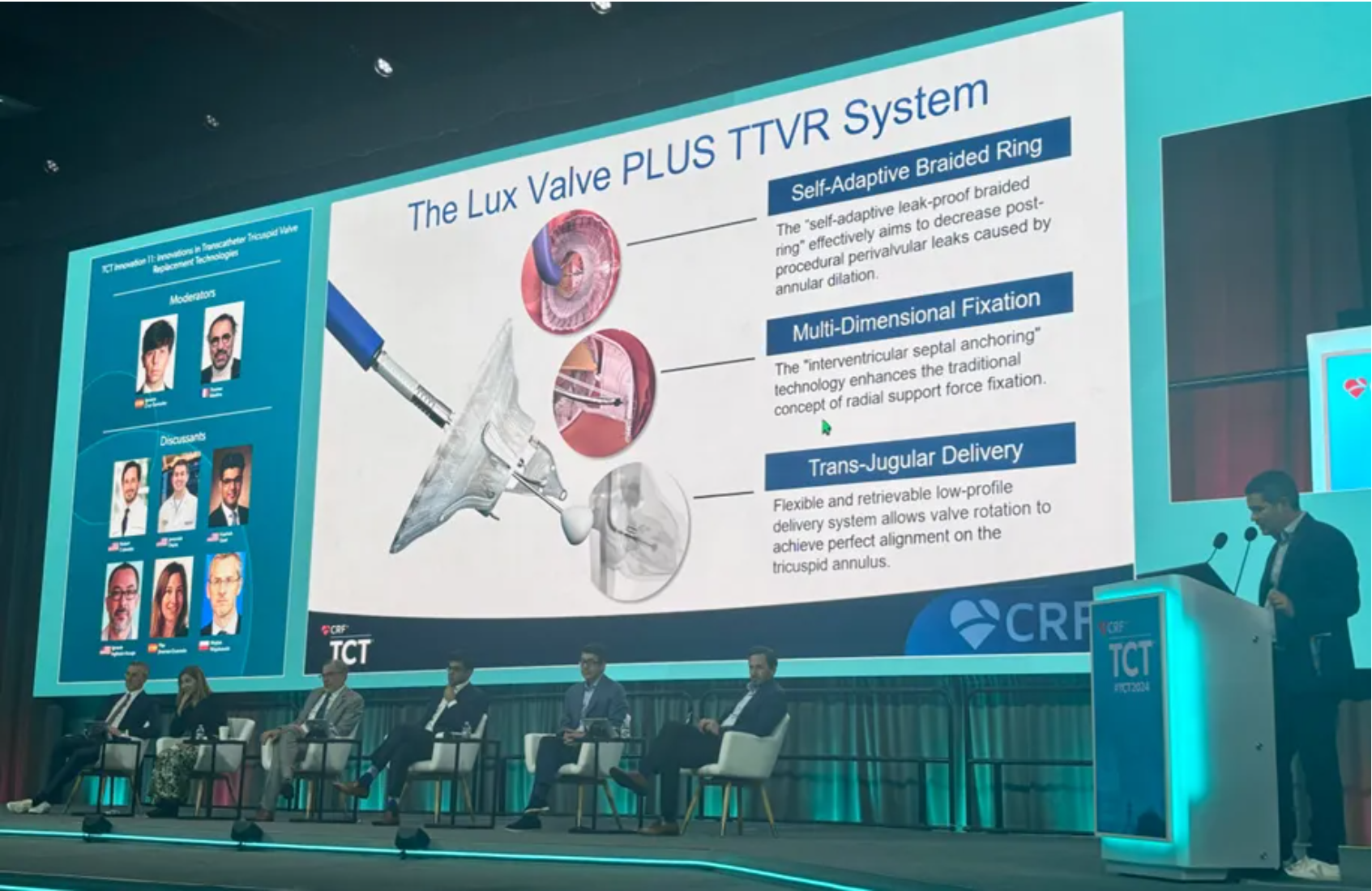
● Global Experiences of Treating TR with LuX-Valve Plus
- U.S.: Local clinical experience is accumulating continuously
- France: A Live Case of LuX-Valve Plus Implantation in Large Anatomy Patient, Partnered by A Joint Team from Four Countries, Broadcasted at AVAM 2024
-Germany: Cutting-Edge Updates on LuX-Valve Plus in Transcatheter Tricuspid Valve Treatment at the 11th Mainz Heart Valve Symposium
- Spain: A Successful Case of LuX-Valve Plus in Patient with Endocarditis was Shared at TCT 2024
- Japan: Experience of the First Implantation of LuX-Valve Plus in Japan was Shared at APCASH 2024
- Brazil: Tansjugular is the Way to Go, Supported by LuX-Valve Plus Implantation Cases
- Hong Kong: Abundant Experiences of LuX-Valve Plus in Challenging Cases were Shared
- Beijing: A Live Broadcast of LuX-Valve Plus Implantation in a Complex Case, Collaboratively Completed by China and Europe
- Chengdu: Jenscare Presented Both the Tricuspid and Mitral Valve Products at PCRCCV 2024
● JensClip Completed the Enrollment for Confirmatory Clinical Trial and Six-month Follow-up
● One-Year Clinical Results of LuX-Valve Plus TRAVEL II Study were Officially Released at TCT 2024 in U.S.
The one-year clinical results of clinical trial TRAVEL II study of LuX-Valve Plus, which was independently developed by Jenscare (9877.HK), were officially published by Prof. Juan F. Granada (Cardiovascular Research Foundation, New York, U.S.) at TCT 2024 in U.S.. This is another significant sharing after the six-month results shared at New York Valves 2024 earlier this year. The astonishing results demonstrated the excellent performance within the unique design and won high attention among the attendances.
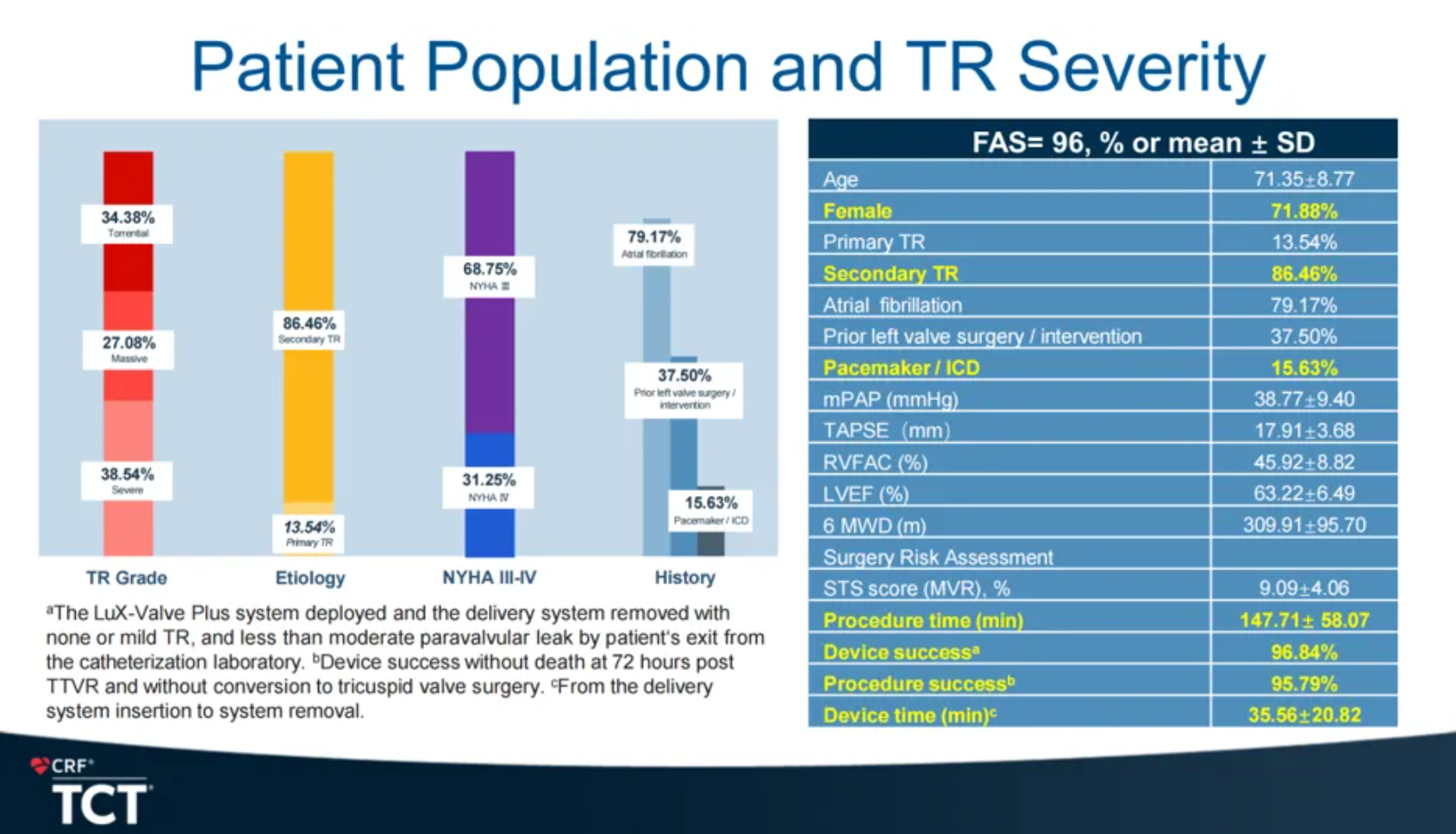
TRAVEL II study primarily aims to evaluate the long-term safety and efficacy of LuX-Valve Plus in the application on patients with severe tricuspid regurgitation (TR). TRAVEL II clinical trial enrolled 96 patients from 15 centers in China. The average age of the patients was 71.35 years old with an average STS score of 9.09%. 37.5% of the patients had prior left heart valve surgery, and 15.63% of them had pacemaker/ICD implanted before. Patients were combined with multi other comorbidities, which formed a poor baseline and increased the risks of surgical treatment or difficulties of other interventional therapies.
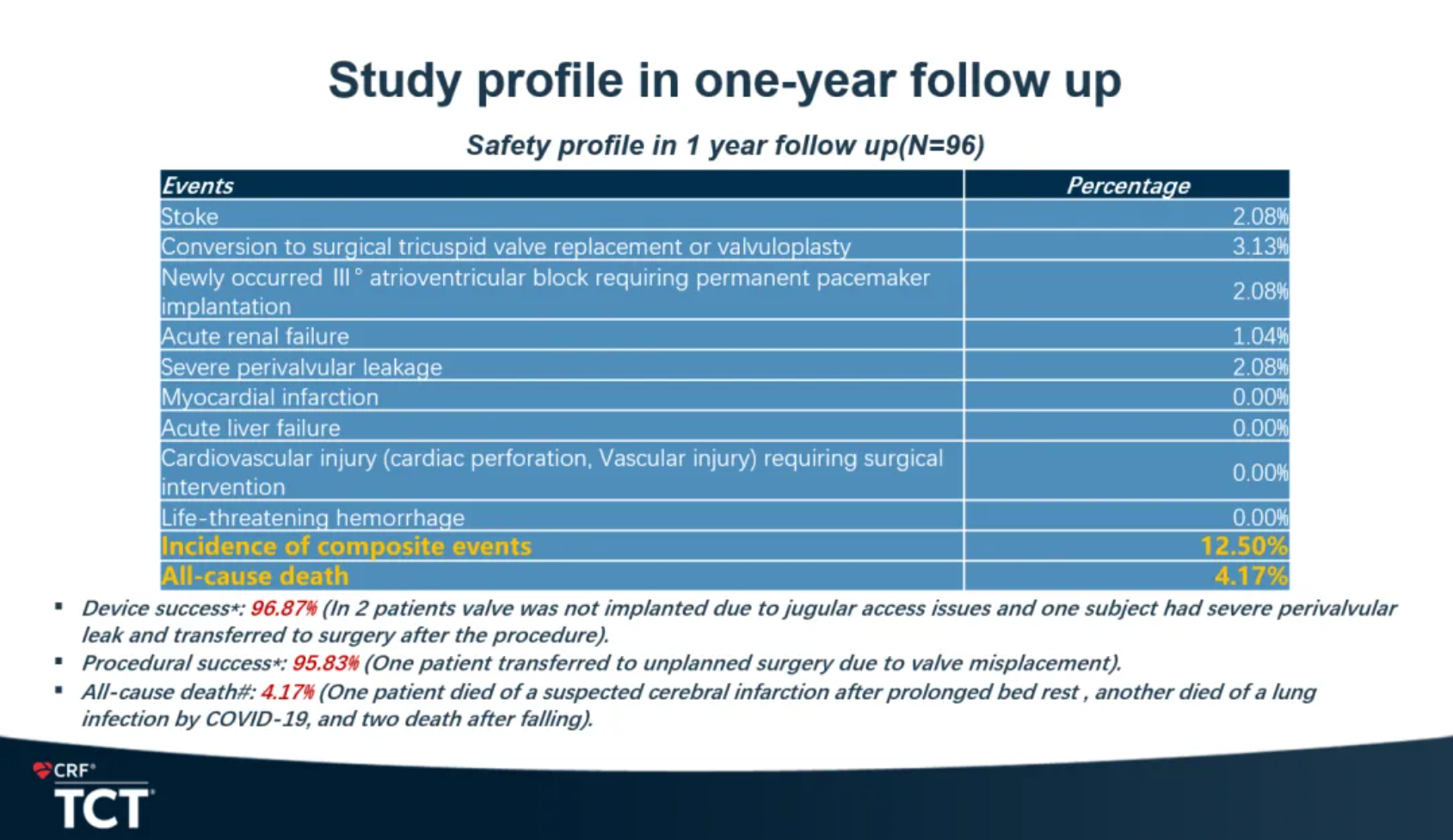
The safety results showed that the incidence of composite events at one year was 12.50% and remained low. Incidence of myocardial infarction, stroke, use of ECMO or IABP, acute liver failure, long-term mechanical ventilation (>72 hours), cardiovascular injury requiring surgical intervention (heart perforation, vascular injury), and life-threatening severe bleeding were all 0%. All-cause mortality was only 4.17%. Incidence of acute renal failure, severe paravalvular leakage, and conversion to surgical tricuspid valve replacement or tricuspid valvuloplasty were 1.04%, 2.08%, and 3.13% respectively. Incidence of new onset degree III AVB requiring permanent pacemaker implantation was only 2.08%.
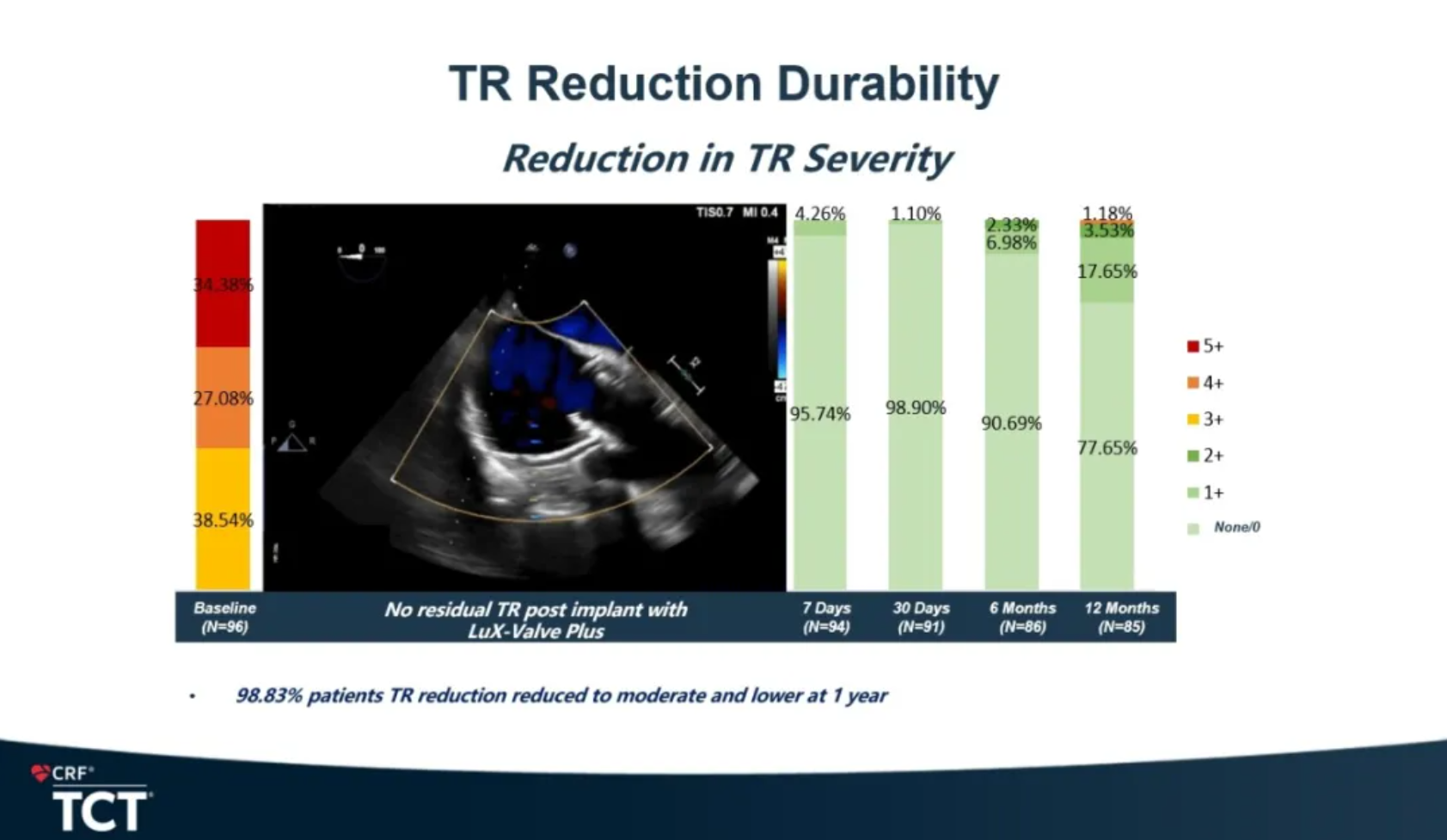
The efficacy results showed that the TR grade, NYHA classification, and QoL (quality of life) improved significantly. 95.30% of patients showed no moderate or above TR at one year. Meanwhile, the reformation of right heart/ventricle benefits as well according to the Echo Core Lab results.
In terms of NYHA cardiac function improvement, 80% of patients improved from pre-procedure NYHA class III/IV to class I/II at 30 days, and 85% improved to NYHA class I/II at one year. In terms of quality of life (QoL), patients increased their Kansas City Cardiomyopathy Questionnaire (KCCQ) averaging score by 15 points at 30 days and increased by 21 points at one year. The results indicate that the cardiac function and QoL of patients improved continuously.
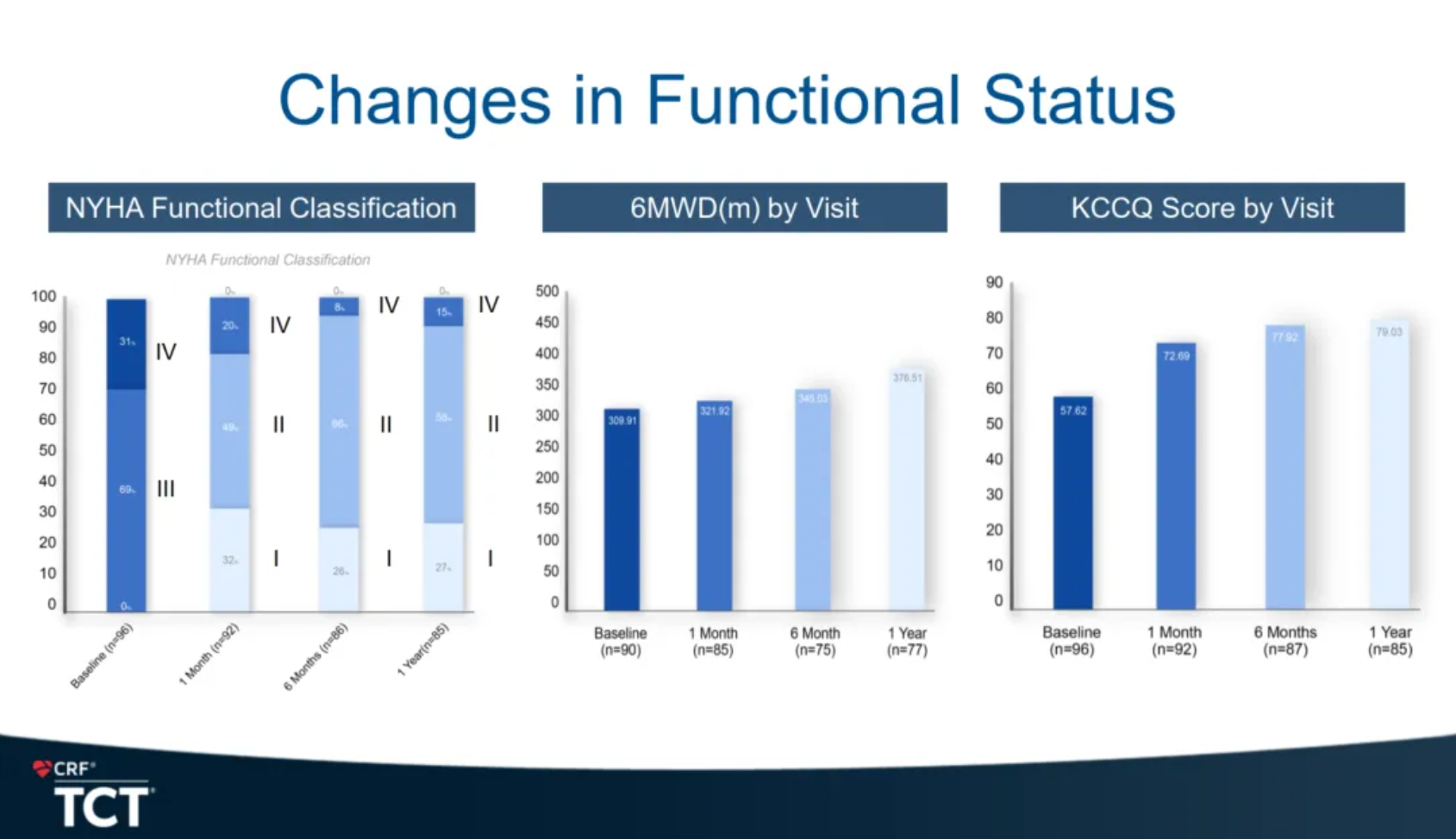
Prof. Granada concluded that LuX-Valve Plus system is a versatile TTVR device that does not depend on radial force for anchoring. Its innovative design (ventricular septal anchor & leaflet-grasping clips) provides an alternative mechanism for anchoring and stability. The multicenter, TRAVEL II study showed that LuX-Valve Plus system is safe and effective in achieving short delivery times, low composite event rates, significant and durable TR reduction and improvement in functional and QoL metrics at one year.

In addition, Dr. Guido Ascione (Cardiovascular Research Foundation, New York, U.S.) conducted a propensity-score matched analysis of one-year clinical outcomes of T-TEER versus TTVR among high-risk patients with symptomatic TR, using the data from LuX-Valve Plus TRAVEL II study as a reference for TTVR. The results showed that TTVR demonstrated a higher procedural success rate in addressing the elimination of TR to T-TEER. Additionally, at one year, there were no statistically significant differences between replacement and repair in clinical outcomes or functional scores.

● One-Year Outcomes of LuX-Valve Plus in Large Anatomy Patients were Shared at PCR London Valves 2024
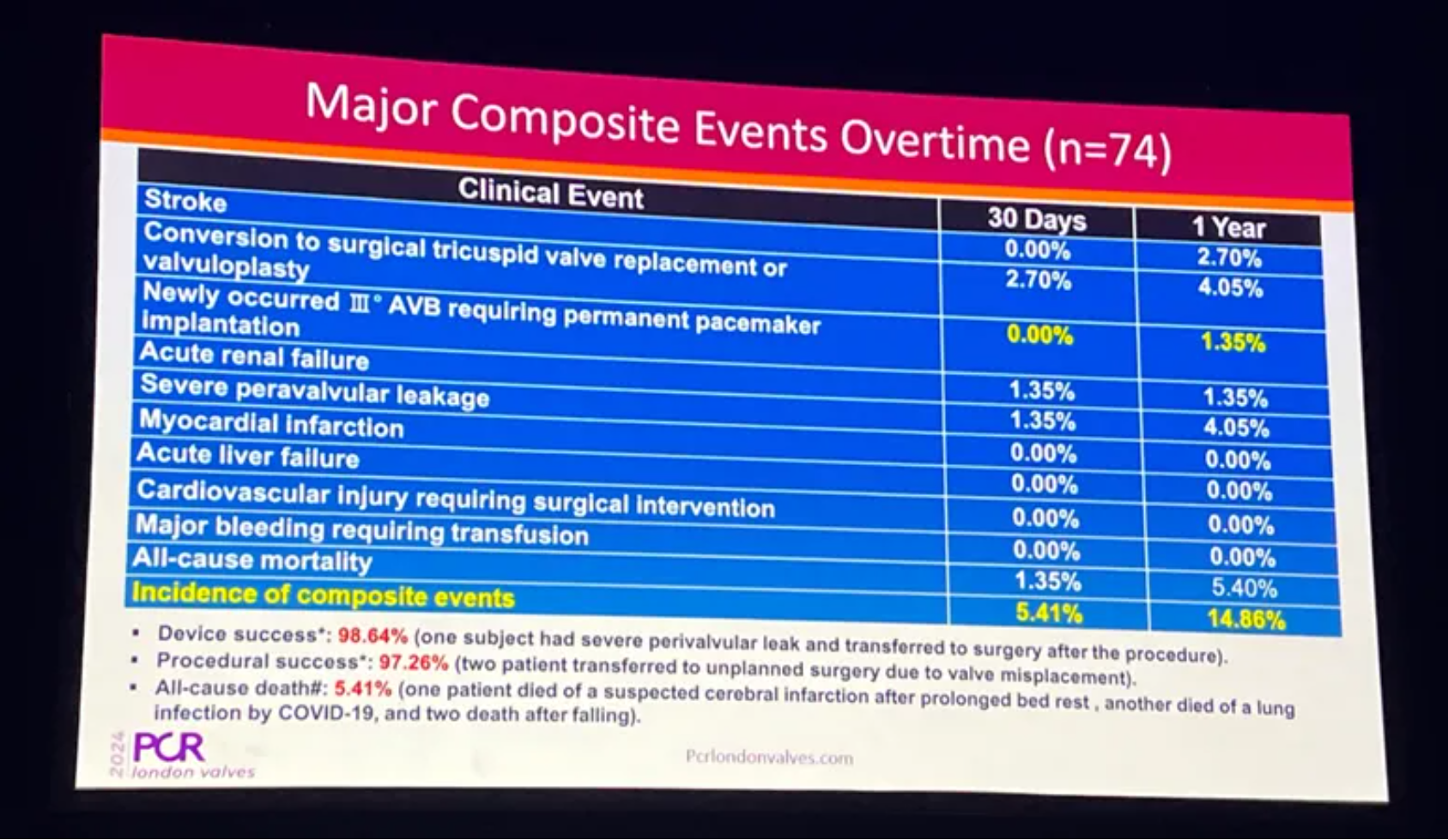
The one-year outcomes of LuX-Valve Plus in large anatomy patients (LAP) were shared by Prof. Thomas Modine (CHU de Bordeaux, Bordeaux, France) at the PCR London Valves 2024 Late-Breaking Trials session. He introduced the overview of more than 400 implantation cases in large anatomy patients all over the world, and particularly combined with the one-year follow-up of patients with annular diameter greater than 50mm from LuX-Valve Plus TRAVEL II Study. Prof. Thomas Modine said he was very happy to see LuX-Valve Plus offers size greater than 50mm. Many of the large anatomy patients are not treated by T-TEER, and many of them are not eligible for the device in current market. It is very important to see how these patients are treated out of scope anatomically. The results showed that the device success rate reached 98.64%, TR grade reduced with durability, and the cardiac function and QoL improved continuously in LAP from TRAVEL II study.

The safety results showed that at one year, LuX-Valve Plus remains low at composite event rates in patients with large anatomies, with only 1.35% of new onset permanent pacemaker implantation and 0% of major bleeding requiring transfusion.
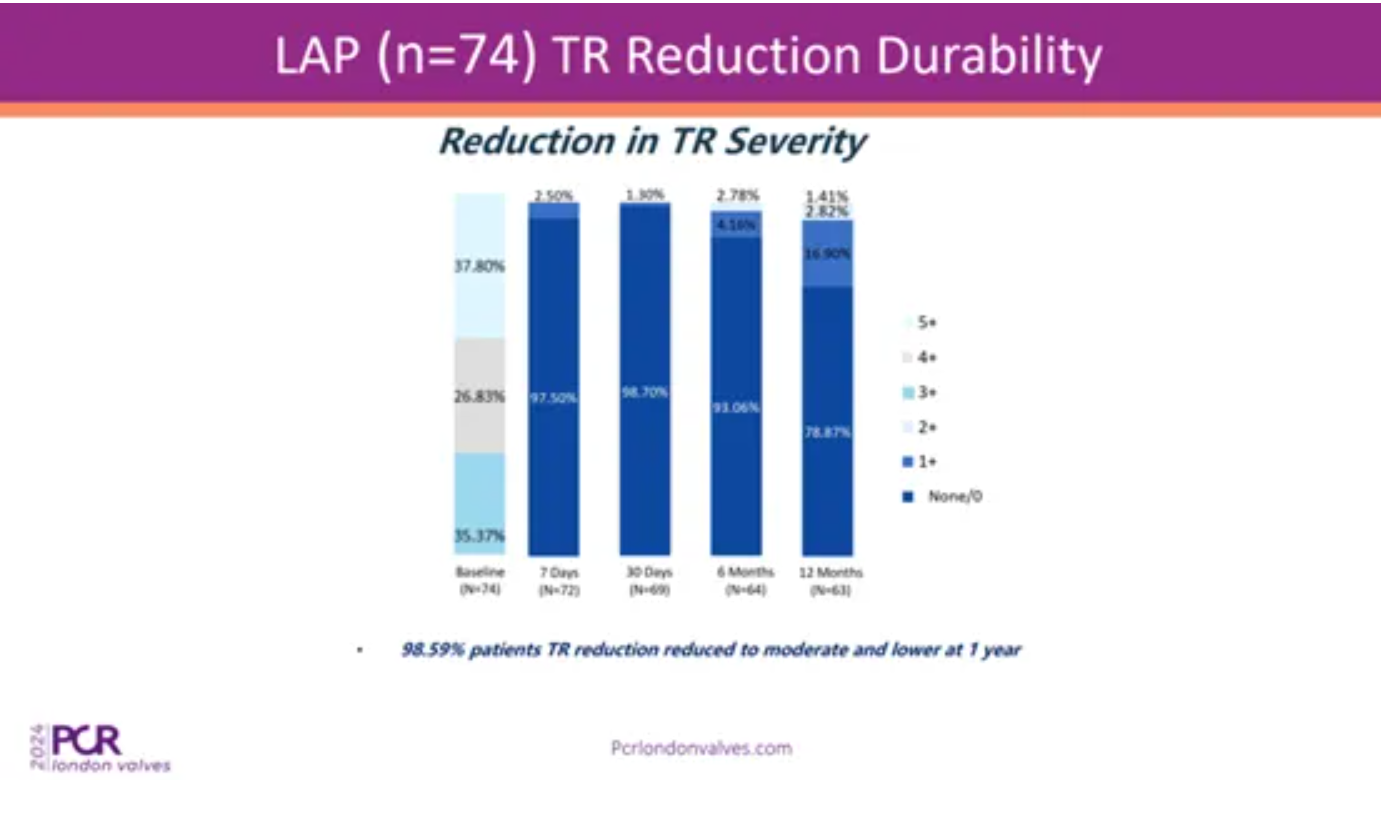
The efficacy results showed that the implantation of LuX-Valve Plus in LAP is very efficient. At one year, 98.59% of the LAP showed TR grade reduced to moderate. Prof. Thomas Modine commented that one importance of TTVR is to see the elimination of TR, not only reduce it. And it is especially challenging in LAP. This is a very good outcome to consider in many patients who are treated today.
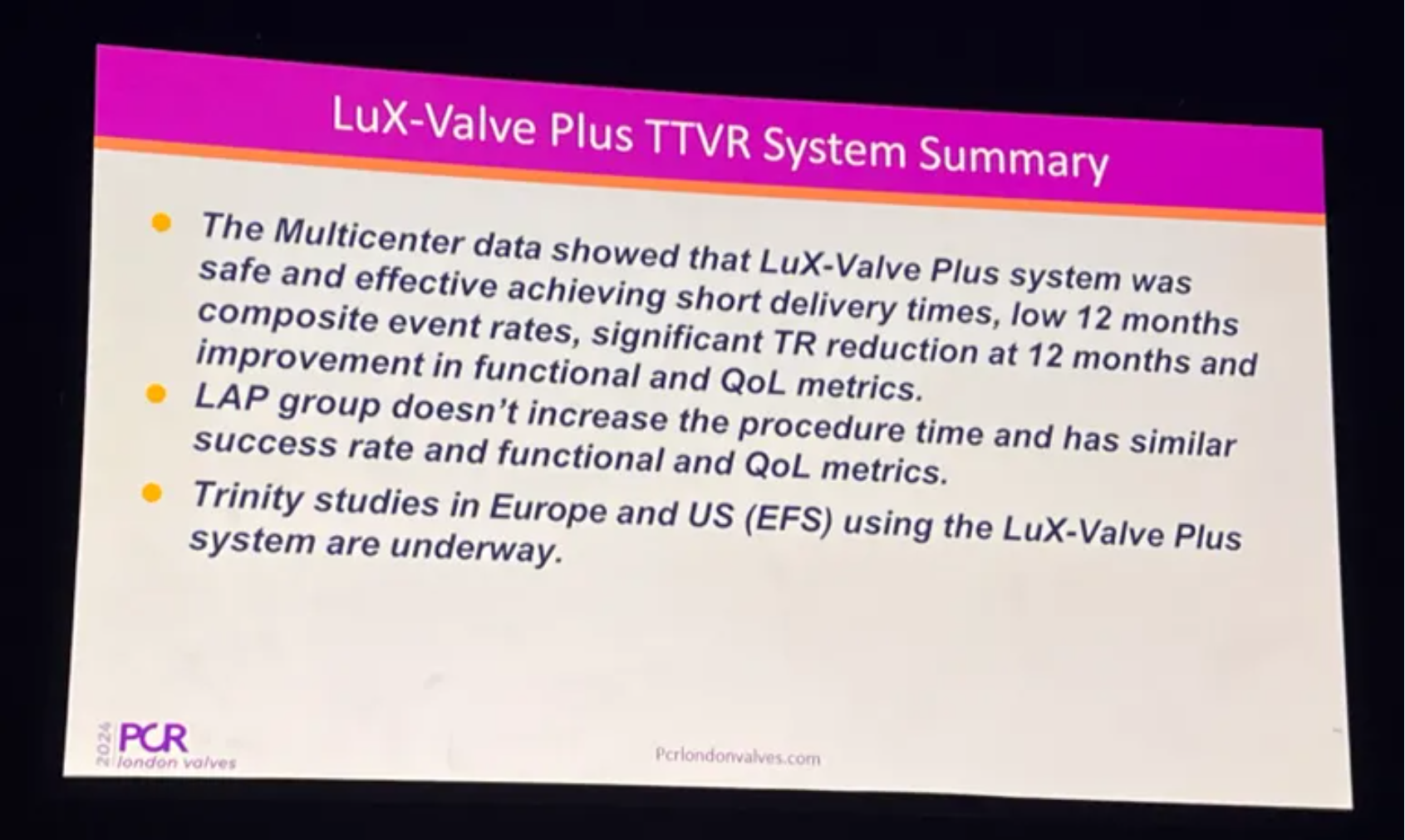
Prof. Thomas Modine summarized that LuX-Valve Plus in LAP showed favorable results. It was safe and effective in achieving short delivery times, low 12 months composite event rates, significant TR reduction and improvement in functional and QoL metrics. LAP group does not increase the procedure time and has similar success rate and functional and QoL metrics. The investigational studies in Europe and U.S. using the LuX-Valve Plus are underway.
Moreover, many other physicians from all over the world shared their experiences and study results of using LuX-Valve Plus. A professor from France shared his excellent results of LuX-Valve Plus implanting in patients with large anatomy during the investigational study in Europe. A professor from spain brought a recorded video of LuX-Valve Plus implantation and introduced the procedure in details. A professor from Hong Kong, China shared his experience of treating patients had preoperative pacing leads with LuX-Valve Plus. A professor from Brazil added that the unique design of LuX-Valve Plus which does not depend on radial force, could lead to a low incidence of postoperative pacemaker implantation.
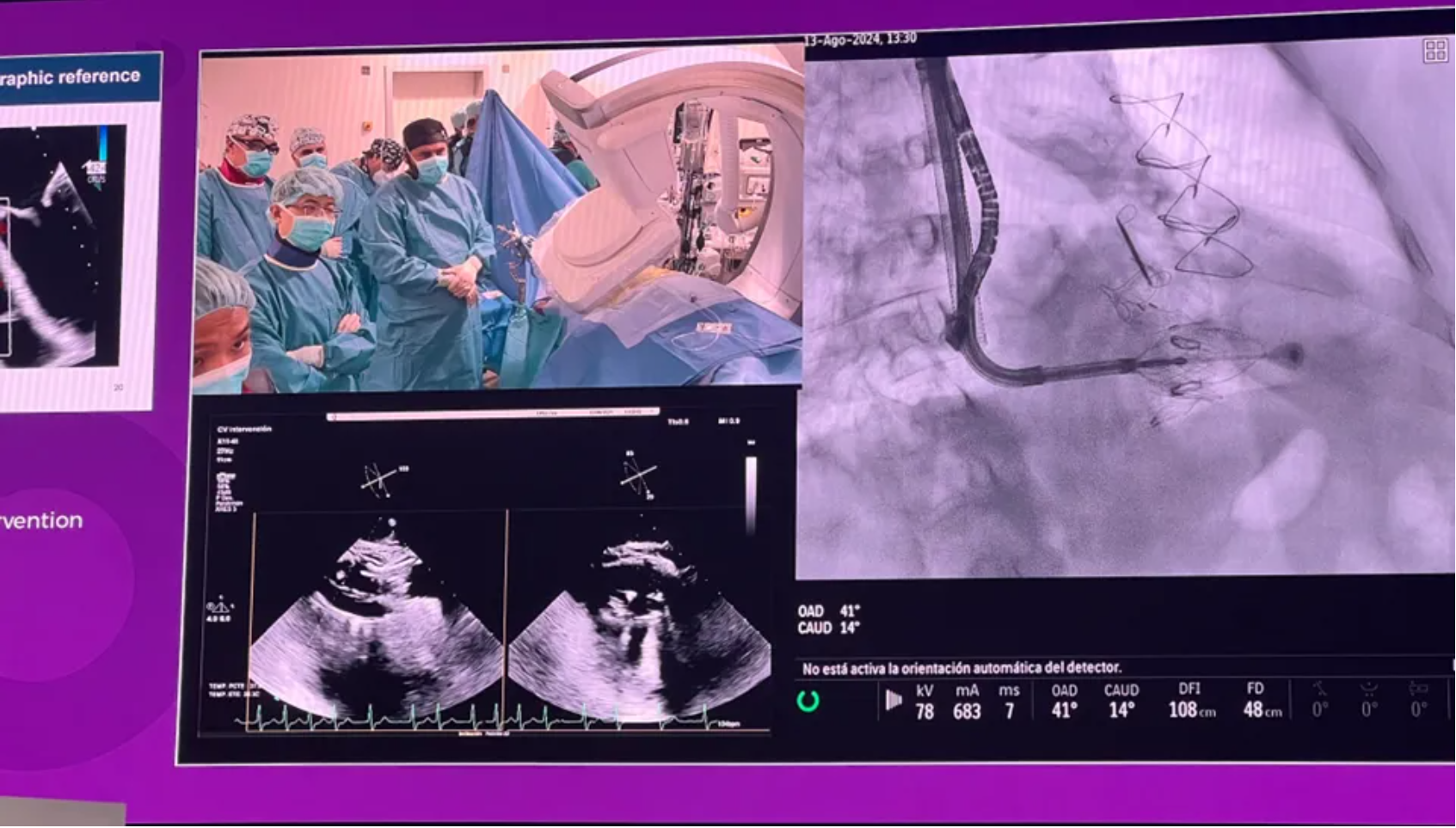
● Global Experiences of Treating TR with LuX-Valve Plus
U.S.: Local clinical experience is accumulating continuously
LuX-Valve Plus is under investigational study in the U.S.. Local clinical experience is accumulating continuously.
France: A Live Case of LuX-Valve Plus Implantation in Large Anatomy Patient, Partnered by A Joint Team from Four Countries, Broadcasted at AVAM 2024
During the tricuspid valve session of the AVAM 2024 (Atrio Ventricular Academy Meeting 2024), co-hosted by CHU de Bordeaux and PCR, physicians and professors from China, France, Canada, and U.S. collaboratively performed an international tricuspid valve intervention operation using LuX-Valve Plus, marking a joint effort by four countries.

This case involved a 70-year-old male patient with a large annulus, classified as NYHA Class II. He had undergone aortic valve replacement and mitral valve repair three years earlier and had a pacemaker implanted, with a Tri-Score of 14%. Preoperative cardiac CT assessment showed an average tricuspid annulus perimeter derived diameter of 58.9 mm and a maximum diameter of 62.2 mm. The procedure was successfully completed. Intraoperative transesophageal echocardiography (TEE) confirmed that LuX-Valve Plus successfully grasped the leaflets, achieved proper septal anchor positioning, and maintained stable anchoring of the prosthetic valve overall.

At the end of the session, a professor from France remarked that he was delighted to have successfully performed another large-diameter tricuspid valve replacement operation. He expressed his appreciation for the unique 60-70 mm size range offered by LuX-Valve Plus. With the progression of the European registration clinical trials, significant experience has been accumulated in implanting valves sized 60 mm and above. He hopes that the product would soon achieve CE certification, enabling it to benefit the large number of patients who currently lack effective treatment options due to the unavailability of large valves. Taking today’s patient as an example, traditional TEER therapy and annuloplasty presented significant challenges, making TTVR an excellent choice at this circumstance.

Germany: Cutting-Edge Updates on LuX-Valve Plus in Transcatheter Tricuspid Valve Treatment at the 11th Mainz Heart Valve Symposium
During the 11th Mainz Heart Valve Symposium, co-hosted by the University Medical Center Mainz, the German Cardiac Society (DGK), and PCR Tricuspid Focus Group, a German professor delivered a presentation titled “Transcatheter Tricuspid Valve Replacement Device Overview”. In his talk, he expressed high recognition and praise for LuX-Valve Plus and its unique anchoring mechanism.

According to the professor, an internationally innovative device for transcatheter treatment of TR, is currently undergoing investigational study in Europe aimed at CE Mark approval. He expressed his delight that the safety and efficacy of LuX-Valve Plus are being progressively validated. The 30-day all-cause mortality rate was only 1.08% (n=93). Due to its non-radial force design, the incidence of new-onset degree III AVB requiring permanent pacemaker was just 2.16%. Additionally, the novel transjugular approach demonstrates unique advantages. The study results further revealed that 0% of life-threatening massive bleeding events occurred at 30 days follow-up.
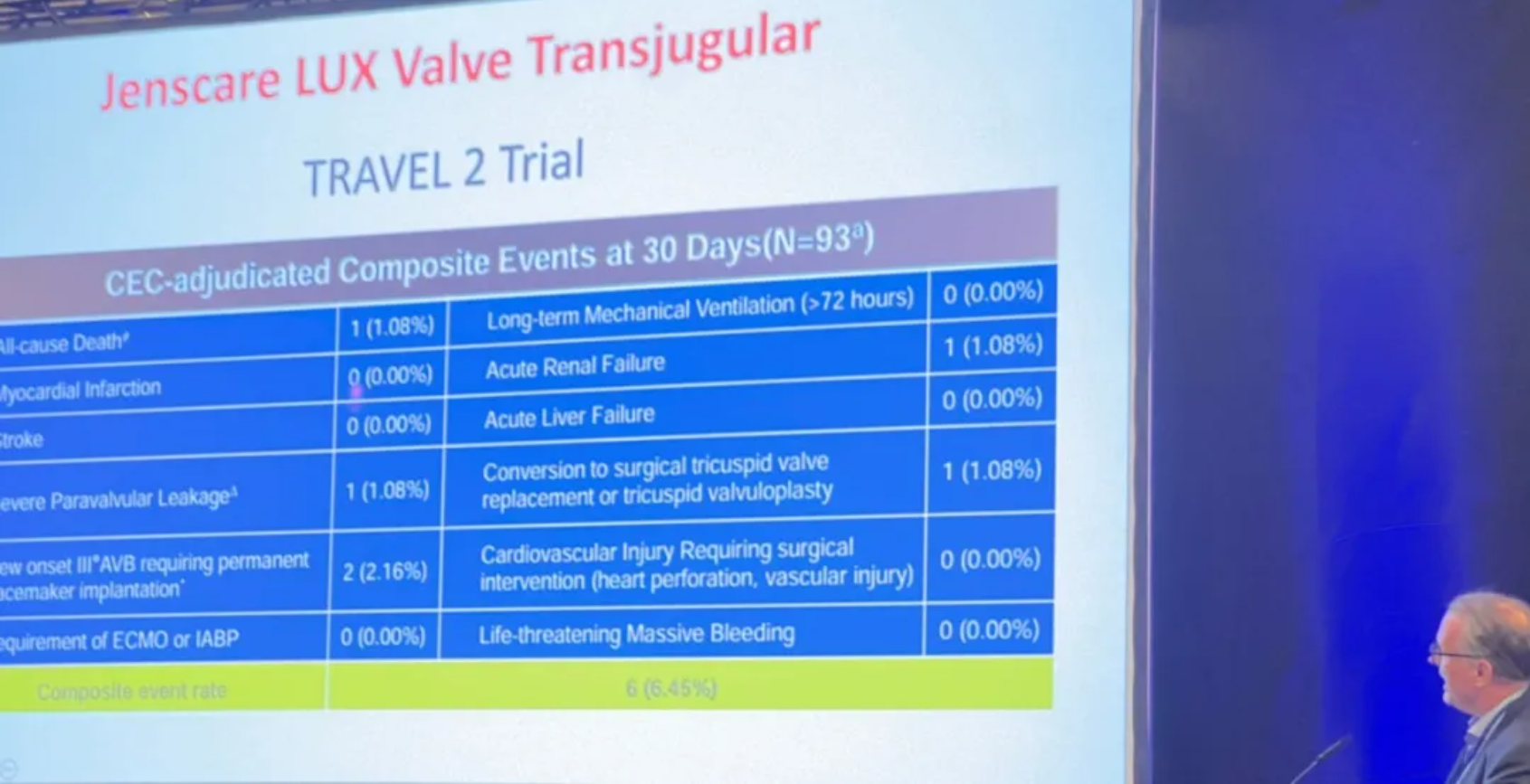
In addition, the professor shared two real cases collaboratively completed with a French professor and explained it in details. Supported by multidimensional imaging techniques such as ultrasound and DSA, the operation team skillfully and precisely implanted LuX-Valve Plus. The device operation time was only 30 minutes, and the intraoperative results were remarkable: immediate disappearance of TR after valve implantation, no significant paravalvular or central regurgitation observed under Doppler ultrasound, normal pulmonary artery pressure, and normal function of the prosthetic valve. Postoperatively, both patients remained stable and were safely transferred back to their wards.
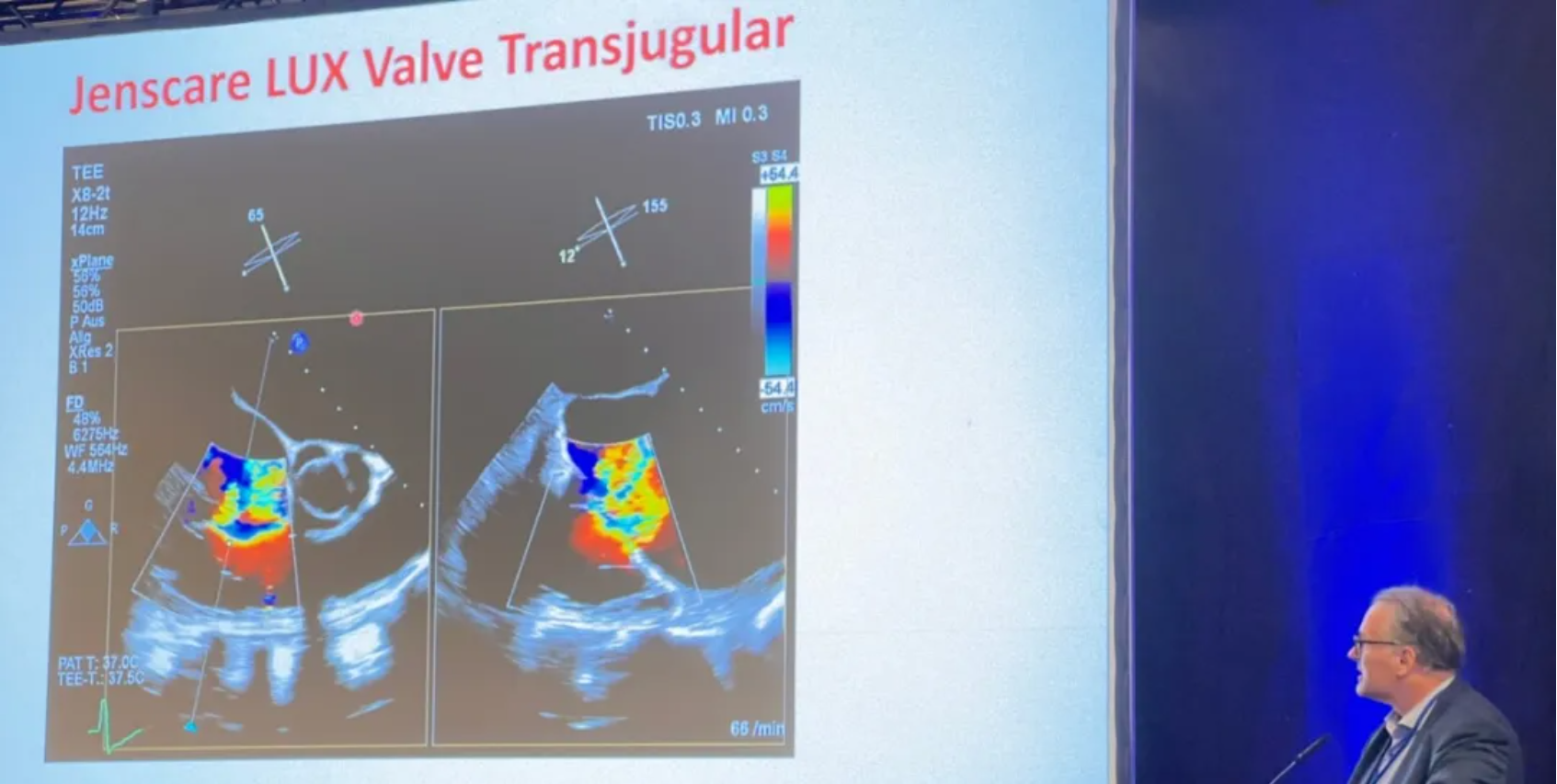
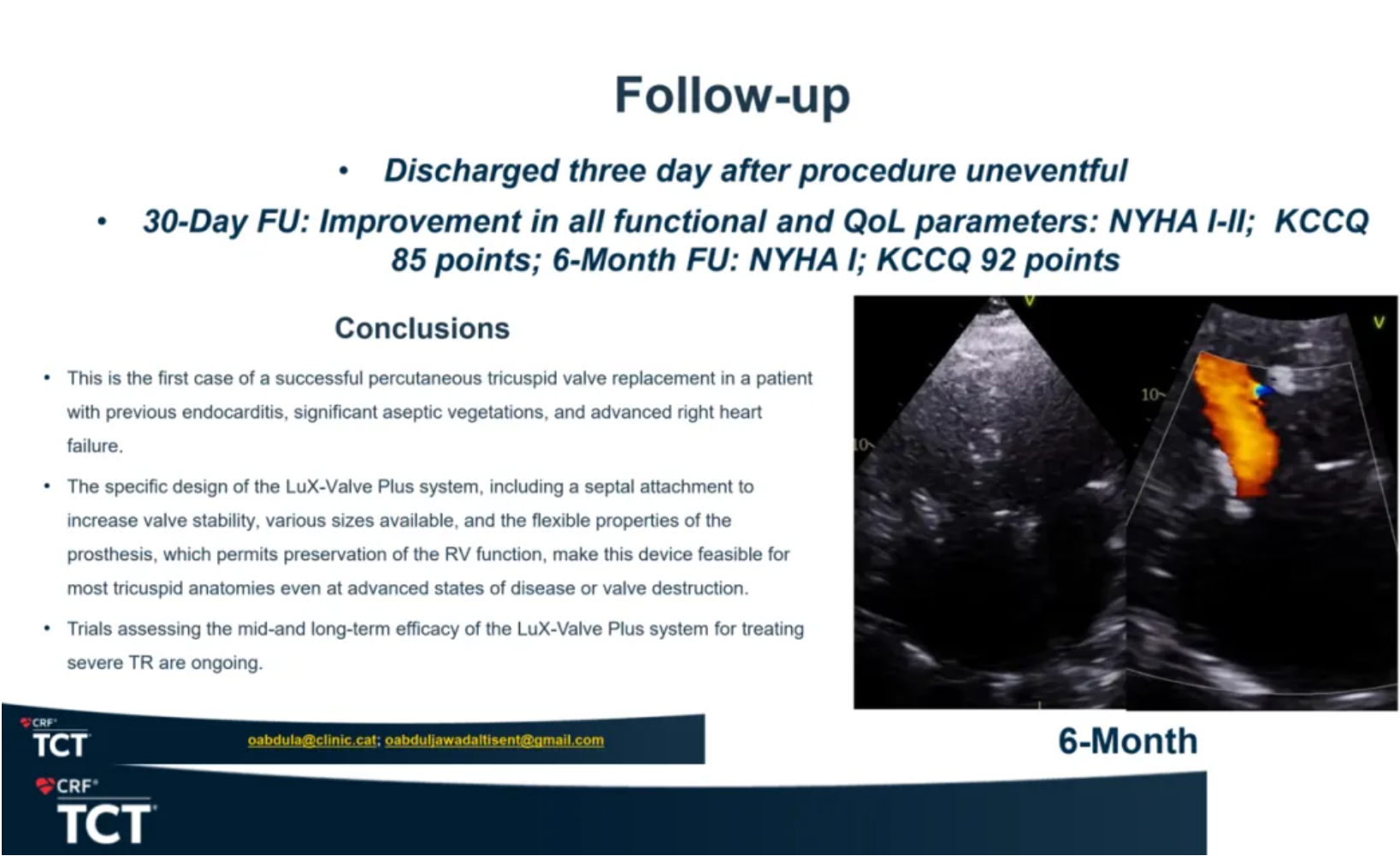
Spain: A Successful Case of LuX-Valve Plus in Patient with Endocarditis was Shared at TCT 2024
A physician came from Spain presented a case report at TCT 2024. A 59 years old male patient with obesity, type A hepatitis, permanent atrial fibrillation and tricuspid endocarditis complicated with tricuspid regurgitation, right heart failure, pulmonary septic embolism and pulmonary empyema requiring long stay at ICU. The physician introduced that the team decided to apply LuX-Valve Plus system after processing the algorithm to treat TR due to the high risk for open heart surgery and unfavorable anatomy for repairment (significant dilation of the tricuspid annulus plus a significant gap) after CT assessment.
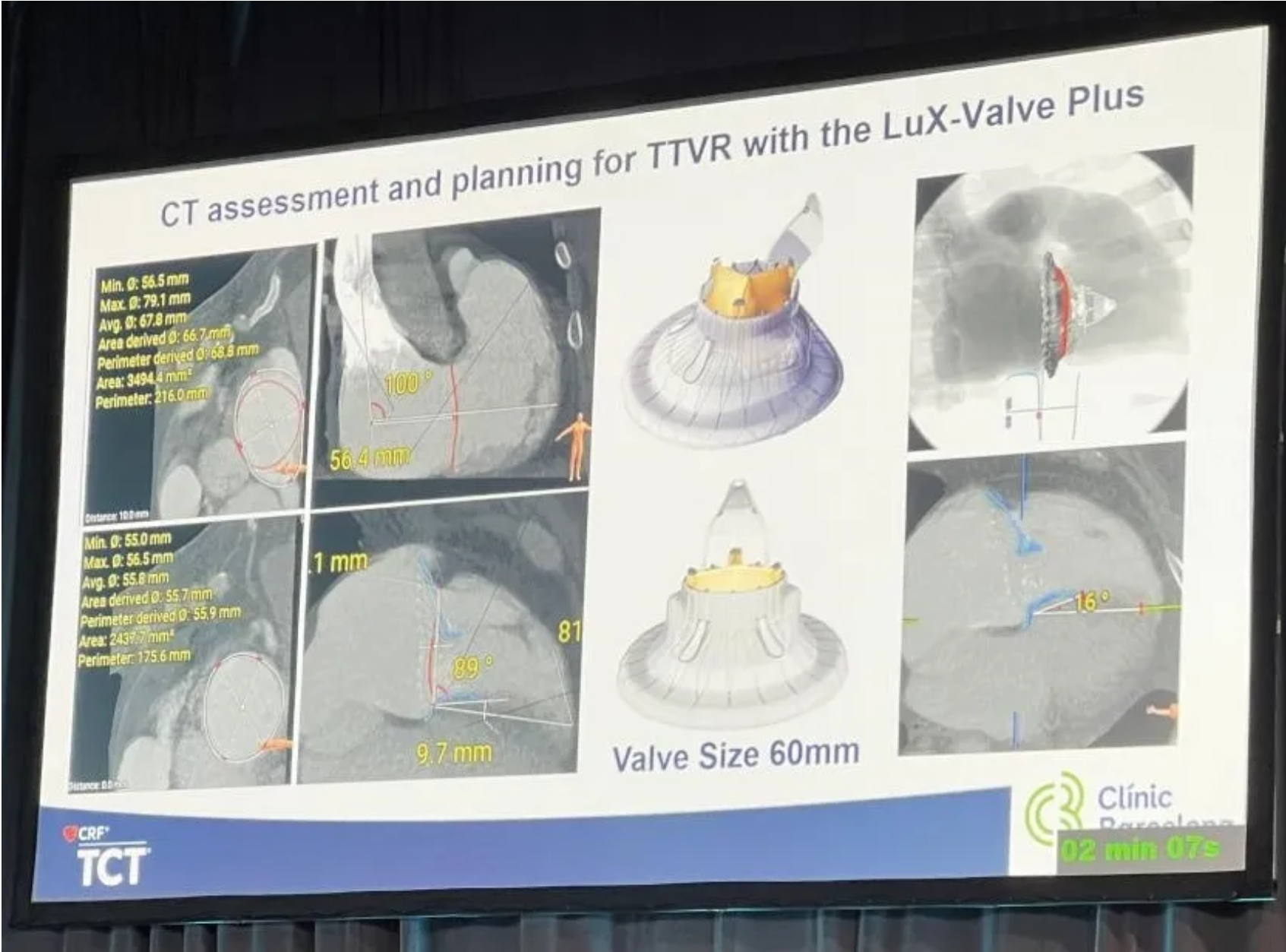
The follow-up showed an improvement in all functional and QoL parameters. The NYHA classification improved to class I/II and class I from preoperative class III at 30 days and six months respectively. KCCQ score increased by 37 points and by 42 points at 30 days and six months respectively. In the end, he concluded that this is a successful case of a percutaneous tricuspid valve replacement in a patient with previous endocarditis, significant aseptic vegetations and advanced right heart failure. The specific design of the LuX-Valve Plus system, including a septal attachment to increase valve stability, various sizes available, along with the flexible properties of the prothesis which permits preservation of the RV function makes this device feasible for most of tricuspid anatomies even at advanced states of the disease or valve destruction.
Japan: Experience of the First Implantation of LuX-Valve Plus in Japan was Shared at APCASH 2024
At APCASH 2024 in Hong Kong, China, during the session titled "Transcatheter Valve Replacement for the Forgotten Valve - The New Frontier", a physician from Japan shared a LuX-Valve Plus case collaboratively performed with Professor Xi Su (Wuhan Asian Heart Hospital, Wuhan, China). The presentation provided a detailed demonstration of the procedural steps and technical highlights of TTVR.

This is the first implantation of LuX-Valve Plus in Japan, said by the Japanese physician. He further appraised that LuX-Valve Plus is expected to become a game-changer in Japan.

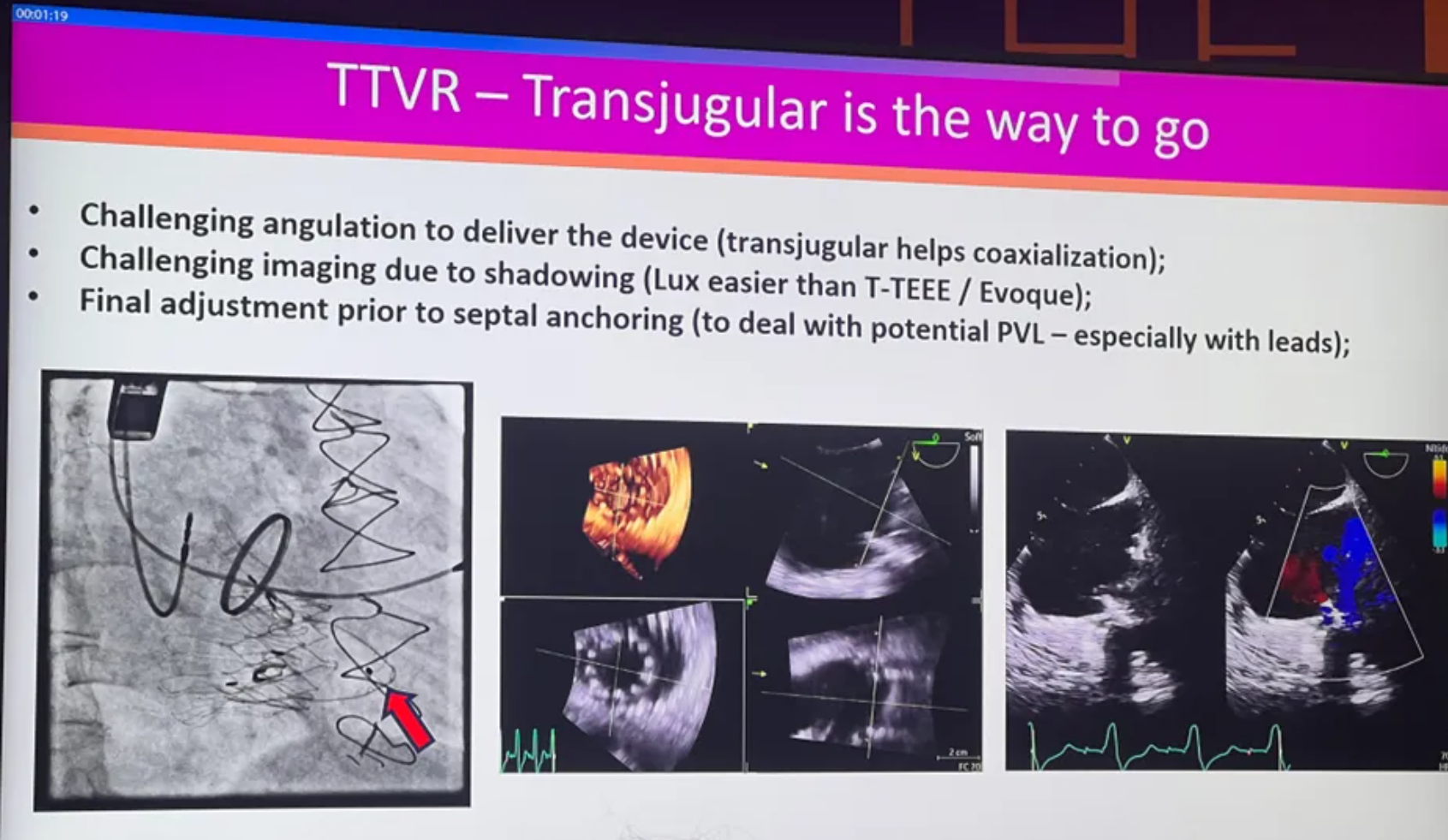
Brazil: Tansjugular is the Way to Go, Supported by LuX-Valve Plus Implantation Cases
At PCR London Valves 2024, a professor from Brazil discussed the advantages of the transjugular approach in TTVR by combining two cases of LuX-Valve Plus implantation.

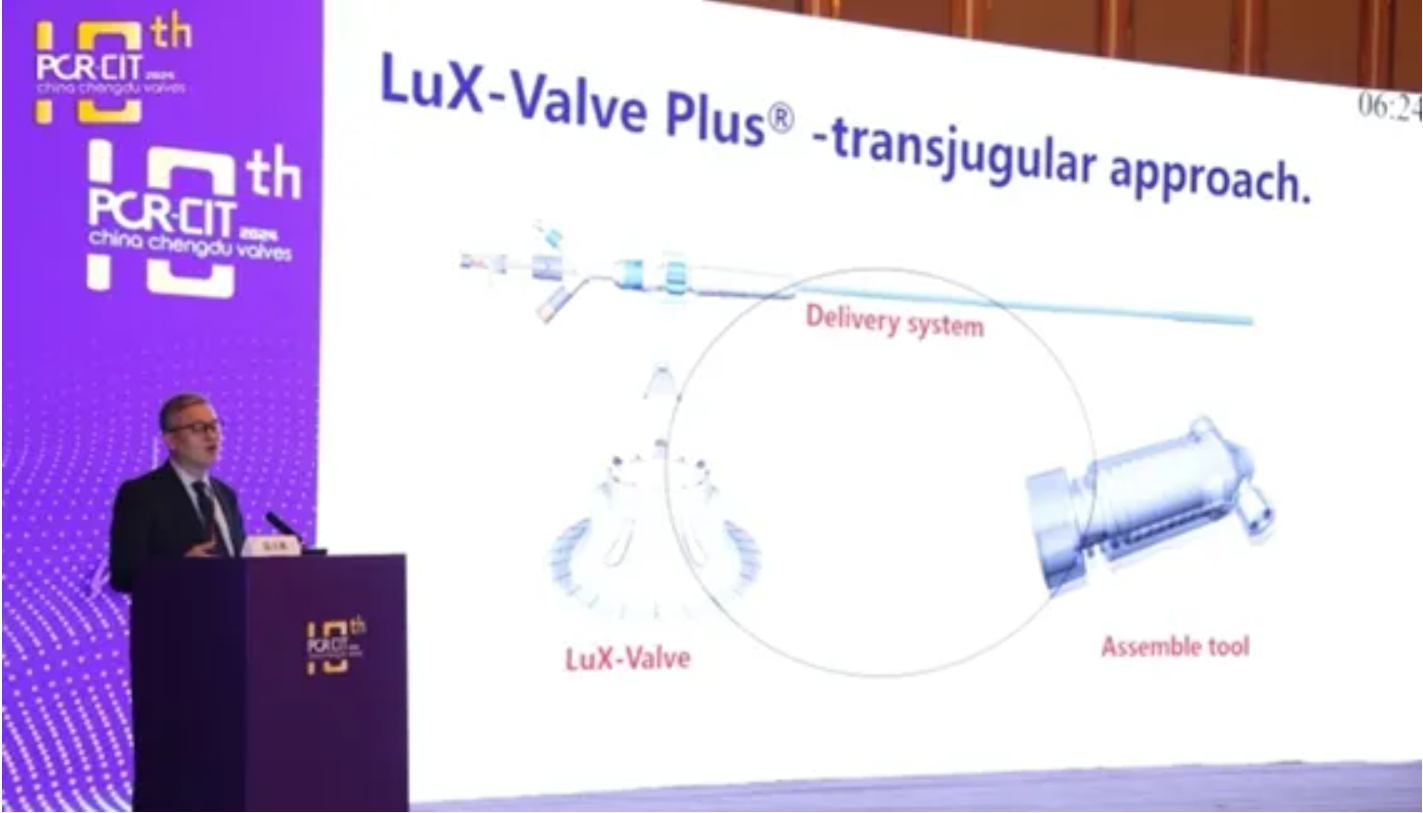
Comparing the transfemoral approach with the transjugular approach, he stated that the transjugular approach, due to its shorter pathway, is expected to reduce surgical risks, shorten operation time, and significantly decrease postoperative complications. It also facilitates easier adjustment of the valve's axial alignment, thereby reducing the occurrence of paravalvular leakages. Moreover, as the transjugular approach does not generate more vascular complications, an increasing number of tricuspid valve replacement devices are exploring this approach, showing a possible future trend for tricuspid valve replacement. As an early explorer of transjugular TTVR devices, LuX-Valve Plus has accumulated abundant clinical experiences.
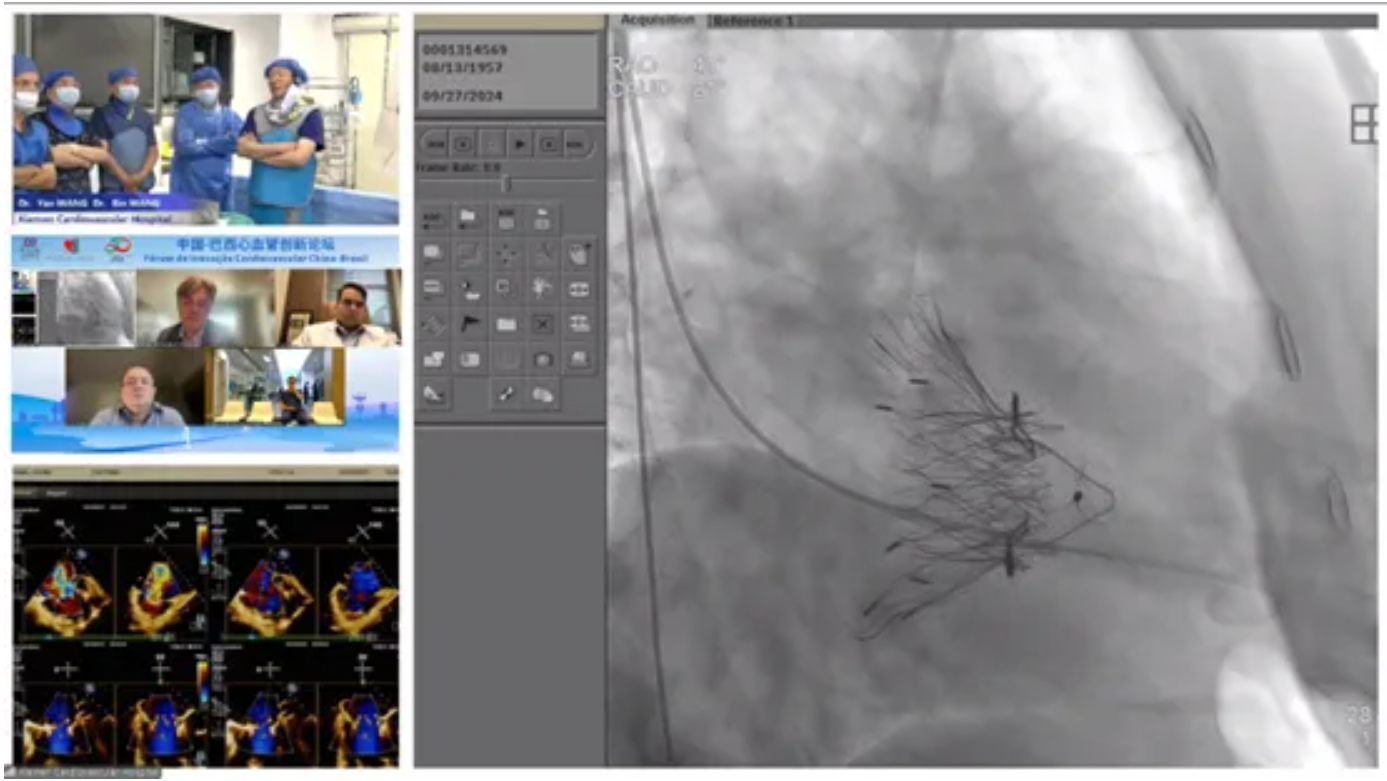
Additionally in October 2024, to celebrate the 50th anniversary of the establishment of diplomatic relations between China and Brazil and to promote medical exchanges and cooperation among BRICS countries, the China-Brazil Cardiovascular Innovation Forum was established. The Brazilian team, after observing the live operation of LuX-Valve Plus, also expressed their admiration. The Brazilian experts praised the procedure and expressed their hope to introduce the system to Brazil to provide new clinical solutions for Brazilian patients with TR. A Brazilian physician commented that throughout the entire process, the delivery system of LuX-Valve Plus entered very smoothly. Moreover, the large leaflet gap in this case posed a significant challenge for traditional TEER therapy and annuloplasty techniques, making tricuspid valve interventional replacement an excellent choice.
Hong Kong: Abundant Experiences of LuX-Valve Plus in Challenging Cases were Shared
At TCT 2024, a physician from Hong Kong, China presented a challenging case of tricuspid valve replacement operation in a patient with existing pacemaker lead. With the assistance of intracardiac echocardiography (ICE), their team successfully completed the operation. They concluded that the innovative design of LuX-Valve Plus can achieve a high rate of successful implantation, especially its compatibility with pre-existing pacemaker leads.
Later at PCR London Valves 2024, a professor from Hong Kong, China shared his experience with TTVR treatment for patients with SLDA. Transcatheter tricuspid valve edge-to-edge repair often fails to achieve good operative outcomes due to patients' anatomies and imaging, and even results in SLDA. The unique anchoring design of LuX-Valve Plus provides high error tolerance in such patients, making it a viable option for TTVR in SLDA patients.

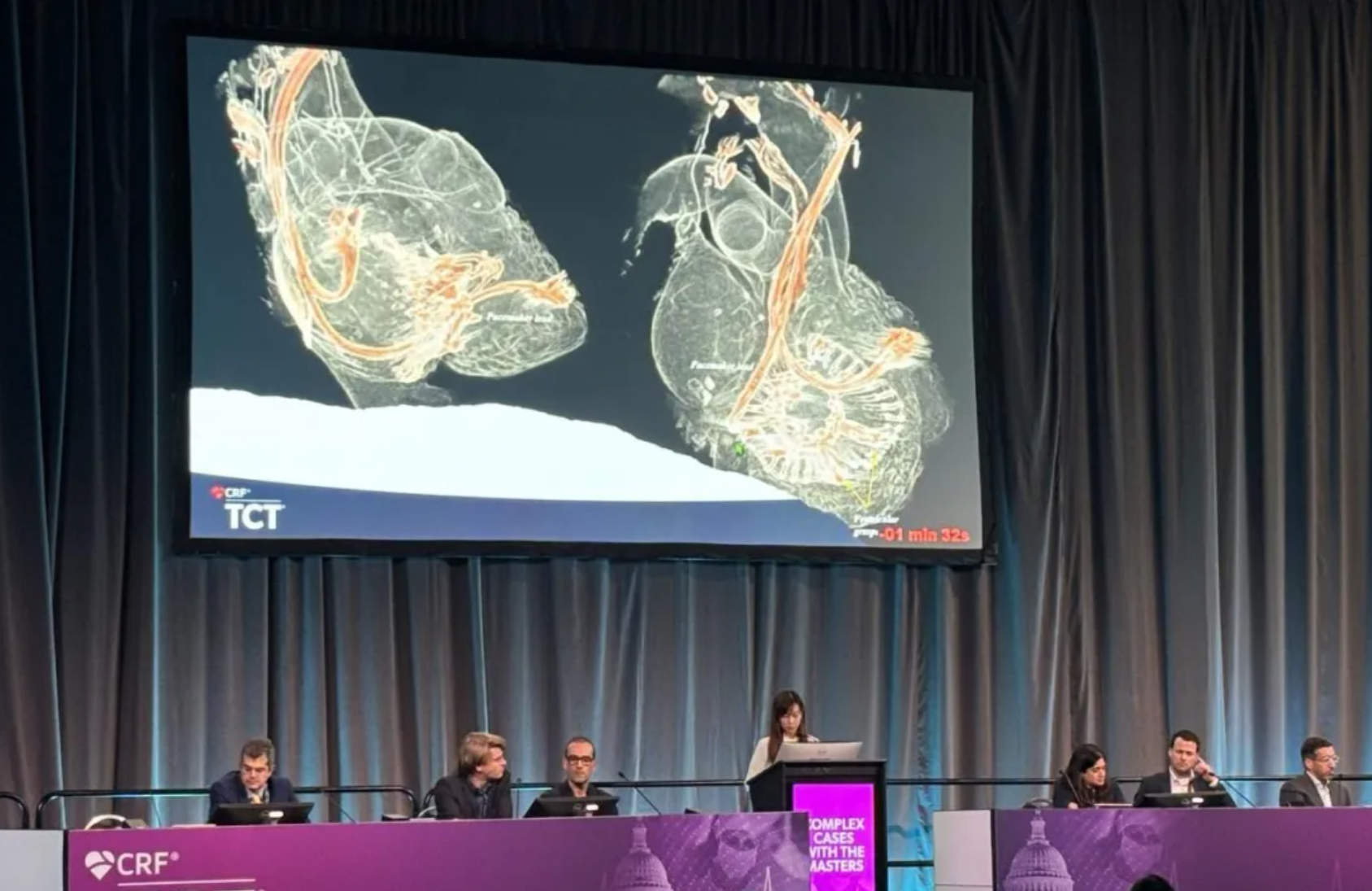
Beijing: A Live Broadcast of LuX-Valve Plus Implantation in a Complex Case, Collaboratively Completed by China and Europe
During the Beijing Valves 2024, Professor Thomas Modine and Professor Lu Fanglin (First People's Hospital of Shanghai, Shanghai, China) together successfully performed a transjugular tricuspid valve interventional replacement operation on a patient with extremely high surgical risk and complex anatomical structure. The patient had poor basic health conditions with an STS score of 12.9%, indicating an extremely high surgical risk, and had a history of pacemaker lead infection removal, causing irreversible iatrogenic damage to the tricuspid valve leaflets, with leaflet detachment and chordal rupture, making T-TEER impossible. After discussion with the team and overseas experts, the operation team decided to use a 30-55 model of LuX-Valve Plus.

Immediately after the operation, transesophageal echocardiography showed that the prosthetic valve was properly positioned, with the bovine pericardial leaflets moving well, opening and closing normally, and no significant regurgitation was observed at the paravalvular or leaflet coaptation sites. The device operation time was only 30 minutes during this broadcasting. Immediately after the valve implantation, TR disappeared, and there was no significant paravalvular leak or central regurgitation under Doppler ultrasound. The pulmonary artery pressure was normal, the artificial valve movement was normal, and the patient was safely returned to the ward within one hour after the operation.
Chengdu: Jenscare Presented Both the Tricuspid and Mitral Valve Products at PCRCCV 2024
At the 2024 Chengdu International Conference on Interventional Treatment of Heart Valve Diseases (PCRCCV 2024), Jenscare presented its TTVR system LuX-Valve Plus and TMVr system JensClip. Professor Feng Yuan (West China Hospital of Sichuan University, Chengdu, China) presented a "LuX-Valve Plus TTVR Recorded Case" under the global attention of structural heart surgeons and engaged in a heated discussion on strategies and techniques with experts present. Professor Lu Fanglin introduced the unique design of LuX-Valve Plus comprehensively. "The interventricular septum anchoring is a major innovation in the field of tricuspid regurgitation interventional treatment, addressing the difficulty of anchoring in the native anatomy of the tricuspid valve. The non-radial support force product design can effectively solve the potential right coronary occlusion and atrioventricular block encountered in traditional surgeries. The TRAVEL II study conducted in China has shown the encouraging trends achieved by LuX-Valve Plus with the follow-up results, preliminarily verifying its safety and effectiveness."
Moreover, Professor Feng Yuan starting from the clinical practical needs and pain points, showcased the transcatheter mitral valve repair system, JensClip, self-developed by Jenscare, to the attendees. Based on the special anatomical structure of mitral valve with regurgitation, JensClip's innovative "claw-wedge" self-locking mechanism can form a multi-angle secure lock from 30° to 0°, thereby reducing the tension of the valvular structure and more safely and effectively reducing mitral regurgitation. Professor Feng Yuan, combining the experience of the clinical center, said: "The unique diamond linkage structure of JensClip, while capturing freely on both sides/single side, increases the bi-directional retrieval function, which is expected to well avoid chord entanglement and simultaneously improve the safety."

● JensClip Completed the Enrollment for Confirmatory Clinical Trial and Six-month Follow-up
JensClip, the self-developed clip-based TMVr system for treatment of severe mitral regurgitation, has recently completed the enrollment for confirmatory clinical trial and the six-month clinical follow-up with outstanding clinical results.
Previous Release
Jenscare | 2024H1 Newsletter
Disclaimer
The information disclosed in this article is for academic communication purposes only and should not be construed as medical advice under any circumstances. We make no representations or warranties as to the accuracy, completeness or timeliness of all original, reproduced or shared content. For any medical device mentioned in this article, our company does not make any commitment and guarantee on its performance and clinical performance in diagnosis and treatment activities.
The regulatory status of LuX-Valve Plus is as follows: pending NMPA approval in China and in investigational clinical study under FDA and EMA.














